IUI Fail
Treatments
- Endometrial Receptivity Array
- Microbiome Investigation
- ICSI
- IVF
- Blastocyst Transfer
Endometrial Receptivity Array
What is it?
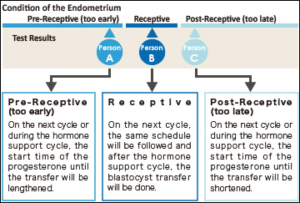 The term ERA stands for Endometrial Receptivity Analysis or Array. In this test, a small sample tissue from endometrial lining (innermost layer of Uterus) is used for evaluating whether the Uterus is ready for implantation of embryo or not.
The term ERA stands for Endometrial Receptivity Analysis or Array. In this test, a small sample tissue from endometrial lining (innermost layer of Uterus) is used for evaluating whether the Uterus is ready for implantation of embryo or not.
In the menstrual cycle of a woman the period from 19th to 23rd days is known as “implantation window” during which, the uterus gets prepared for the implantation process. It is part of the luteal phase and the endocrine part of ovaries is producing progesterone. This progesterone brings about modifications in the uterine wall so that, it gets prepared for receiving the embryo. The process involves formation of certain proteins that make the lining thicker and more receptive. In majority of females (84%) this window occurs at the exact time while in very few females (16%) this window occurs either before or after this period.
The IVF treatment in such cases fails as, the embryo transfer is occurring at wrong time. It is happening at the time, when the implantation window is either yet to open or has already been closed. So, the implantation is failing.
When is it prescribed?
It is prescribed in the patients where recurrent implantation failures are observed.
How is it done?
- A small endometrial tissue is collected and expression level of the gene 238 which determines endometrial receptivity is investigated.
- The technique involves assessing RNA levels in different stages of menstrual cycle. As this test is reproducible, its findings remain the same months after and hence, the test is not required to be repeated.
- This investigation can clearly indicate whether this implantation window of a particular woman is happening at the right time or the embryo transfer needs to be scheduled on the different date to match the window so that maturation of endometrial lining can be synchronized with embryo.
What are the advantages?
- Due to precise identification of Implantation Window, embryo transfer can be appropriately planned and can yield assured results.
- The agony of failed transfer can be got rid of.
- A minor shift by one of two days in embryo transfer can result into pregnancy.
Microbiome Investigation
What is it?
The results demonstrated existence of an endometrial microbiota that is highly stable during acquisition of endometrial receptivity. However, poor reproductive outcomes for in vitro fertilization patients is associated with pathological modification of its profile. This finding provides a novel microbiological dimension to the reproductive process.
How is it done?
Endometrial fluid and vaginal aspirate are investigated for studying the microbial composition. On the basis of these findings distinction was made as a Lactobacillus-dominated microbiota (>90% Lactobacillus spp.) or a non-Lactobacillus-dominated microbiota (<90% Lactobacillus spp.). Although the endometrial microbiota is not regulated hormonally during the acquisition of endometrial receptivity, the presence of a non-Lactobacillus-dominated microbiota in a receptive endometrium was associated with significant decreases in implantation [60.7% vs 23.1% (P = .02)], pregnancy [70.6% vs 33.3% (P = .03)], ongoing pregnancy [58.8% vs 13.3% (P = .02)], and live birth [58.8% vs 6.7% (P = .002)] rates.
When is it recommended?
Uterocervical microbial colonization has been suspected to influence conception rates, with possible causes including an association between cervical microbial species and a pre-existing uterine infection, or colonization of the endometrium or the embryo during transport through the colonized cervix. This kind of microbiota has a significant impact on reproductive outcome even in ART procedures. So, ART failure for no known reason makes it mandatory to examine the Uterocervical microbiota so as to eliminate this as possible reason for ART failures. Thus, the main purpose is to investigate the impact of vaginal microbiome composition on reproductive outcomes within the context of infertility treatments, and the implications this have on assisted reproductive technology procedures.
ICSI
What is it?
It is a laboratory procedure where a single sperm is picked up with a fine glass needle and is injected directly into the egg. This is carried out in the laboratory by experienced embryologists using special equipment.
When is it recommended?
It is recommended in any or more of the following conditions.
- When the sperm count is very low
- When the sperm cannot move properly or are in other ways abnormal
- When sperm has been retrieved surgically from the epididymis (MESA/PESA) or the testes
- (TESE/TESA), from urine or following electro-ejaculation
- When there are high levels of antibodies in the semen
- When there has been a previous fertilisation failure using conventional IVF.
What are the advantages?
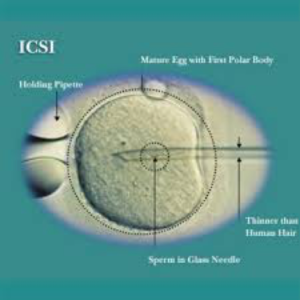 Very few sperms are required for the process and the ability of the sperm to penetrate the egg is no longer important as this has been assisted by the ICSI technique. ICSI does not guarantee that fertilisation will occur as the normal cellular events like fusion of nuclei still need to occur once the sperm has been placed in the egg.
Very few sperms are required for the process and the ability of the sperm to penetrate the egg is no longer important as this has been assisted by the ICSI technique. ICSI does not guarantee that fertilisation will occur as the normal cellular events like fusion of nuclei still need to occur once the sperm has been placed in the egg.
IVF : In Vitro Fertilization
What is it?
During IVF, an egg is removed from the woman’s ovaries and fertilised with sperm in a laboratory. The fertilised egg, called an embryo, is then returned to the woman’s womb to grow and develop. It can be carried out using patient’s eggs and partner’s sperm, or eggs and/or sperm from donors.
When is it recommended?
Offered to women under the age of 43 who have been trying to get pregnant through regular unprotected sex for 2 years, or who have had failed cycles of artificial insemination.
How is it done?
IVF involves 6 main stages:
1. suppressing your natural cycle– the menstrual cycle is suppressed with medication
2. boosting your egg supply– medication is used to encourage the ovaries to produce more eggs than usual
3. monitoring your progress and maturing your eggs– an ultrasound scan is carried out to check the development of the eggs, and medication is used to help them mature
4. collecting the eggs– a needle is inserted into the ovaries, via the vagina, to remove the eggs
5. fertilizing the eggs– the eggs are mixed with the sperm for a few days to allow them to be fertilised
6. transferring the embryo(s)– 1 or 2 fertilised eggs (embryos) are placed into the womb
Once the embryo(s) has been transferred into patient’s womb, she’ll need to wait 2 weeks before taking a pregnancy test to see if the treatment has worked.
Blastocyst Transfer
What is it?
The embryo formation after fertilization involves a number of stages such as 2 cell, 4 cells, 8 cells stage, Morula and so on. Gradually, the cells become distinct as outer cells (Trophoblast) and inner cell mass (embryonic cells). There is a small cavity in between. This stage is called blastocyst. Usually, the protective covering of embryo (Zona Pellucida) is still intact. The blastocyst hatches out of this covering to get implanted into endometrial lining (Innermost covering of uterus).
Using this blastocyst (developed in laboratory) for embryo transfer in IVF technique is called blastocyst transfer (Blast transfer).
When is it recommended?
Blast transfer is recommended for those patients with one more of the following conditions.
- The patients of higher age (35 years or more).
- Repeatedly failed IVF cycles.
- Recipient patients (who are receiving eggs or embryos from donor).
- Those who don’t have the mental preparation for further IVF cycles.
What are the advantages?
- Higher success rates even for patients of higher age.
- Growth pattern of the embryo is established in the laboratory before implantation. So, the doctor has a clear understanding about progress of embryo after implantation.
